ENDOMETRIOSIS & PAIN
RELIEF Study: Managing Endometriosis Pain With At-Home Brain Stimulation

About this study
✅ Randomized & Blinded Clinical Trial
Participants are randomly assigned to receive either active or sham brain stimulation. Neither you nor the study team will know which group you’re in until the study is complete.
⌚️ Daily, At-Home Sessions
You’ll use the NettleEndo device at home for 20 minutes per day, 5 days a week, over 3 months, while answering questions about pain, mood, and sleep.
🎁 Compensation Available
Participants who meet minimum engagement and data-sharing milestones will receive a $50 Amazon gift card.
🤳 Wearable Data Optional
If you have a Fitbit, Apple Watch, Garmin, Oura, or similar device, you can optionally connect it to help researchers explore the relationship between sleep, movement, and symptoms.
What's Involved
This study evaluates whether transcranial direct current stimulation (tDCS), delivered via the NettleEndo device, can help reduce endometriosis-related pelvic pain and improve overall well-being.
You’ll be asked to:
-
Use the device at home for 20 minutes a day, 5 days per week, for 12 weeks
-
Complete short daily surveys on pain, sleep, mood, and activity
-
Fill out occasional questionnaires on anxiety, depression, sleep quality, and quality of life
-
Optionally share data from your wearable device
-
Continue using your existing medications and routines (as long as they remain stable)
Your device will look and feel the same regardless of your group, and it will be programmed to deliver either active or sham stimulation.


Steps to Participate
-
Check Eligibility: Complete a short online screener. You must confirm that you have a verified diagnosis of endometriosis from a medical professional, via imaging or surgery.
-
Enroll & Provide Consent: If eligible, you’ll review the study details and sign an electronic consent form through the Alethios platform.
-
Onboard:
-
Watch a brief training video
-
Confirm receipt of your NettleEndo device
-
Complete baseline assessments and health questionnaires
-
-
Begin Study Participation:
-
Use the NettleEndo device for 20 minutes per day, 5 days a week, for 12 weeks
-
Complete daily and weekly surveys through the Alethios platform
-
If you connect a wearable device, wear it consistently throughout the study
-
-
Maintain Adherence:
-
Adherence is measured through the Alethios platform and the Samphire Clinical App
-
You must complete all scheduled surveys and device sessions to meet adherence
-
If using a wearable, it must be worn on at least 80% of study days
-
-
Return the Device & Close Out:
-
Return your NettleEndo device at the end of the study
-
Adherent participants will receive a $50 Amazon gift card and access to their individual study data
-
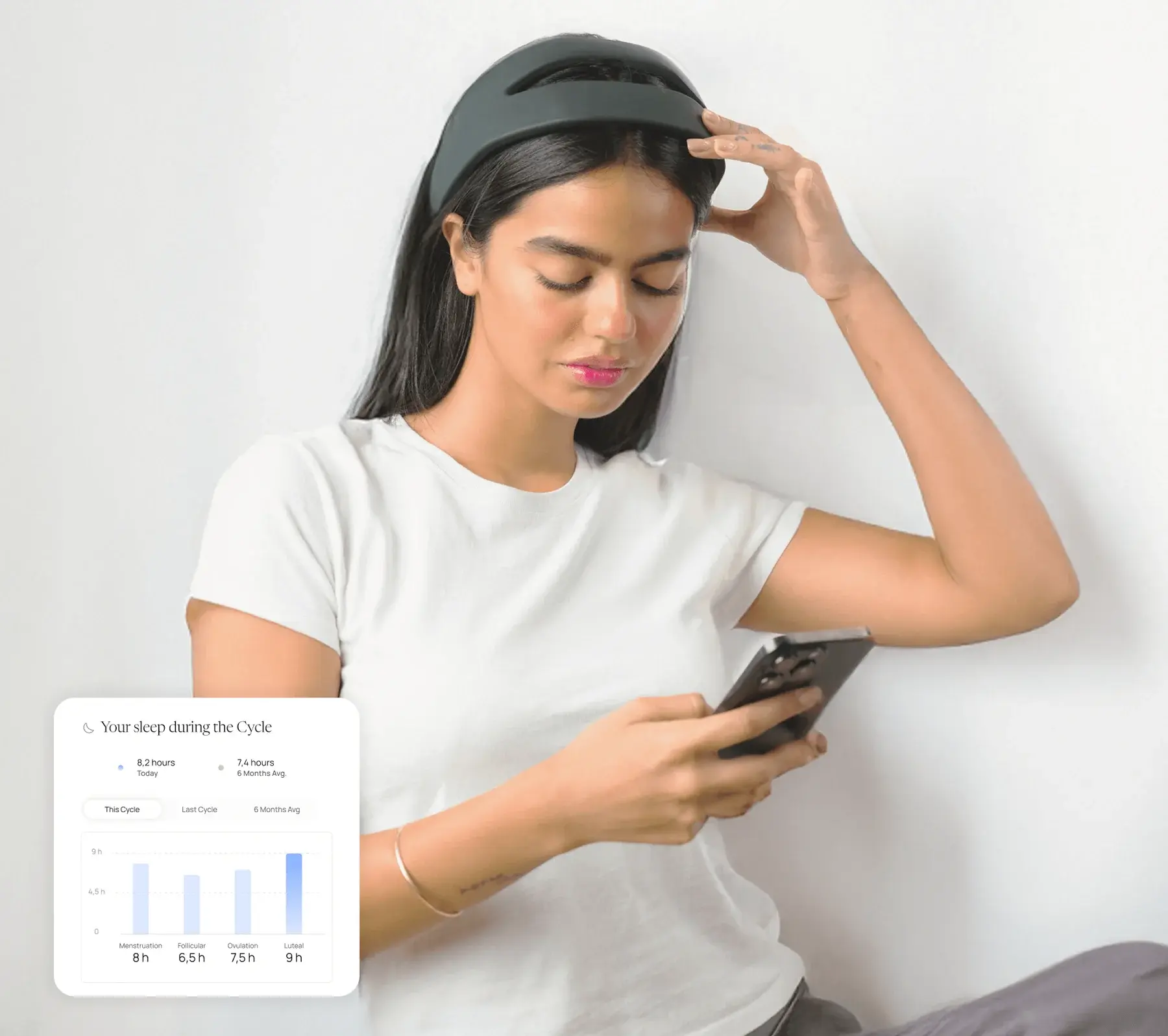
What's being studied?
The NettleEndo device is a non-invasive neuromodulation headband that sends low-level electrical currents to areas of the brain involved in pain perception and mood regulation. The study will assess whether this intervention helps reduce pelvic pain, improve sleep, and enhance emotional well-being in individuals with endometriosis. This study is sponsored by Samphire Neuroscience, the developer of the NettleEndo device.

More about this study
This study is conducted by Samphire Neuroscience in collaboration with researchers and hosted on the Alethios virtual research platform. It is randomized, double-blind, and placebo-controlled. The study is IRB approved, and follows a rigorous clinical protocol with participant privacy, safety, and transparency as top priorities. You’ll be debriefed and unblinded at the end of the study.
See study listing at https://clinicaltrials.gov

Am I eligible?
You may qualify if you:
-
Are between 22 and 45 years old
-
Were assigned female at birth
-
Have a confirmed diagnosis of endometriosis via imaging or surgery
-
Experience chronic pelvic pain (with or outside of menstruation)
-
Have regular menstrual cycles (24–35 days)
-
Are not pregnant, breastfeeding, or planning pregnancy during the study
-
Are not changing your medications or treatments during the study period
-
Have no history of epilepsy, stroke, head trauma, or scalp conditions
-
Do not have metal implants or electronic devices in the head/neck
-
Are not participating in another clinical trial
-
Have access to a smartphone and internet